3D Bioprinted Heart Provides New Tool for Surgeons
Surgeons will soon have a powerful new tool for planning and practice with the creation of the first full-sized 3D bioprinted model of the human heart.
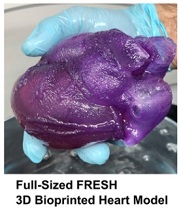
The first full-size 3D bioprinted human heart model using the Freeform Reversible Embedding of Suspended Hydrogels (FRESH) technique has been created by Carnegie Mellon University’s (CMU’s) Adam Feinberg, PhD, and his team. The model, created from MRI data using a specially built 3D printer, realistically mimics the elasticity of cardiac tissue and sutures. This milestone represents the culmination of two years of research, holding both immediate promise for surgeons and clinicians, as well as long-term implications for the future of bioengineered organ research.
The FRESH technique of 3D bioprinting was invented in Dr. Feinberg’s lab to fill an unfilled demand for 3D printed soft polymers, which lack the rigidity to stand unsupported as in a normal print. FRESH 3D printing uses a needle to inject bioink into a bath of soft hydrogel, which supports the object as it prints. Once finished, a simple application of heat causes the hydrogel to melt away, leaving only the 3D bioprinted object.
While Dr. Feinberg, a professor of biomedical engineering and materials science and engineering at CMU and an affiliated faculty member of the McGowan Institute for Regenerative Medicine, has proven both the versatility and the fidelity of the FRESH technique, the major obstacle to achieving this milestone was printing a human heart at full scale. This necessitated the building of a new 3D printer custom made to hold a gel support bath large enough to print at the desired size, as well as minor software changes to maintain the speed and fidelity of the print.
Major hospitals often have facilities for 3D printing models of a patient’s body to help surgeons educate patients and plan for the actual procedure; however, these tissues and organs can only be modeled in hard plastic or rubber. Dr. Feinberg’s team’s heart is made from a soft, natural polymer called alginate, giving it properties similar to real cardiac tissue. For surgeons, this enables the creation of models that can cut, suture, and be manipulated in ways similar to a real heart. Dr. Feinberg’s immediate goal is to begin working with surgeons and clinicians to fine tune their technique and ensure it’s ready for the hospital setting.
“We can now build a model that not only allows for visual planning, but allows for physical practice,” says Dr. Feinberg. “The surgeon can manipulate it and have it actually respond like real tissue, so that when they get into the operating site they’ve got an additional layer of realistic practice in that setting.”
The paper, published in ACS Biomaterials Science and Engineering, represents another important marker on the long path to bioengineering a functional human organ. Soft, biocompatible scaffolds like that created by Dr. Feinberg’s group may one day provide the structure onto which cells adhere and form an organ system, placing biomedicine one step closer to the ability to repair or replace full human organs.
“While major hurdles still exist in bioprinting a full-sized functional human heart, we are proud to help establish its foundational groundwork using the FRESH platform while showing immediate applications for realistic surgical simulation,” added Eman Mirdamadi, lead author on the publication and former student of Dr. Feinberg.
The research paper was also co-authored by Dr. Feinberg’s students Joshua W. Tashman, Daniel J. Shiwarski, PhD, and Rachelle N. Palchesko, PhD.
Illustration: The Feinberg Lab. Carnegie Mellon University.
Read more...
Carnegie Mellon University News Release (11/18/20)
ACS News (11/18/20)
EurekAlert! (11/18/20)
Chemical & Engineering News (11/18/20)
MedicalXpress (11/18/20)
CNet (11/18/20)
New Atlas (11/19/20)
The Engineer (11/19/20)
Advanced Science News (11/20/20)
SciTechDaily (11/22/20)
Medgadget (11/23/20)
Wired (11/23/20)
Bio: Dr. Adam Feinberg
Abstract (ACS Biomaterials Science and Engineering, 10/23/2020, 6, 11, 6453–6459.)
The FRESH technique of 3D bioprinting was invented in Dr. Feinberg’s lab to fill an unfilled demand for 3D printed soft polymers, which lack the rigidity to stand unsupported as in a normal print. FRESH 3D printing uses a needle to inject bioink into a bath of soft hydrogel, which supports the object as it prints. Once finished, a simple application of heat causes the hydrogel to melt away, leaving only the 3D bioprinted object.
While Dr. Feinberg, a professor of biomedical engineering and materials science and engineering at CMU and an affiliated faculty member of the McGowan Institute for Regenerative Medicine, has proven both the versatility and the fidelity of the FRESH technique, the major obstacle to achieving this milestone was printing a human heart at full scale. This necessitated the building of a new 3D printer custom made to hold a gel support bath large enough to print at the desired size, as well as minor software changes to maintain the speed and fidelity of the print.
Major hospitals often have facilities for 3D printing models of a patient’s body to help surgeons educate patients and plan for the actual procedure; however, these tissues and organs can only be modeled in hard plastic or rubber. Dr. Feinberg’s team’s heart is made from a soft, natural polymer called alginate, giving it properties similar to real cardiac tissue. For surgeons, this enables the creation of models that can cut, suture, and be manipulated in ways similar to a real heart. Dr. Feinberg’s immediate goal is to begin working with surgeons and clinicians to fine tune their technique and ensure it’s ready for the hospital setting.
“We can now build a model that not only allows for visual planning, but allows for physical practice,” says Dr. Feinberg. “The surgeon can manipulate it and have it actually respond like real tissue, so that when they get into the operating site they’ve got an additional layer of realistic practice in that setting.”
The paper, published in ACS Biomaterials Science and Engineering, represents another important marker on the long path to bioengineering a functional human organ. Soft, biocompatible scaffolds like that created by Dr. Feinberg’s group may one day provide the structure onto which cells adhere and form an organ system, placing biomedicine one step closer to the ability to repair or replace full human organs.
“While major hurdles still exist in bioprinting a full-sized functional human heart, we are proud to help establish its foundational groundwork using the FRESH platform while showing immediate applications for realistic surgical simulation,” added Eman Mirdamadi, lead author on the publication and former student of Dr. Feinberg.
The research paper was also co-authored by Dr. Feinberg’s students Joshua W. Tashman, Daniel J. Shiwarski, PhD, and Rachelle N. Palchesko, PhD.
Illustration: The Feinberg Lab. Carnegie Mellon University.
Read more...
Carnegie Mellon University News Release (11/18/20)
ACS News (11/18/20)
EurekAlert! (11/18/20)
Chemical & Engineering News (11/18/20)
MedicalXpress (11/18/20)
CNet (11/18/20)
New Atlas (11/19/20)
The Engineer (11/19/20)
Advanced Science News (11/20/20)
SciTechDaily (11/22/20)
Medgadget (11/23/20)
Wired (11/23/20)
Bio: Dr. Adam Feinberg
Abstract (ACS Biomaterials Science and Engineering, 10/23/2020, 6, 11, 6453–6459.)
